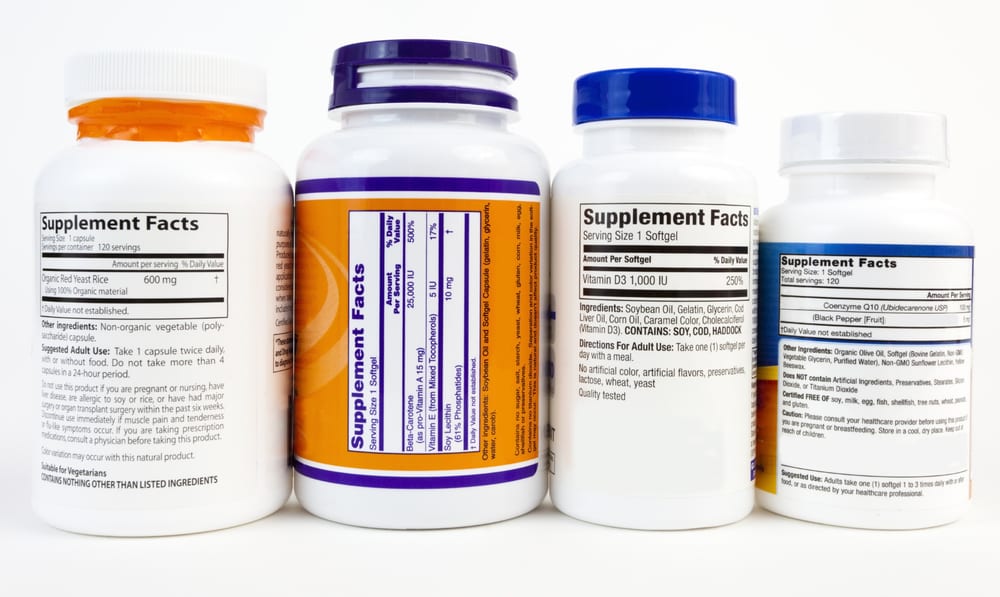
In the near future, consumers of dietary supplements will see changes to labels on their favorite vitamins, minerals, protein powders, and extracts. In order to help consumers understand these changes, CRN (The Council for Responsible Nutrition) launched a Be Label Wise campaign (www.BeLabelWise.org). CRN’s campaign explains the new FDA label guidelines and offers several resources for consumers and industry insiders.
The site has a two-minute video that reviews the Supplement Facts label and introduces the changes. What’s more, the site also has other helpful educational tools like an Infographic, a section entitled ‘How to Read a Supplement Facts Label’, a Fact Sheet, and Tips for Consumers (1). Consumers and industry insiders can access the entire toolkit at https://belabelwise.org/stakeholder-toolkit/.
The Time for Change
As a leader in the supplement industry, CRN applauds the FDA’s upcoming label changes. Brian Wommack, senior vice president, communications, CRN says:
The ‘Supplement Facts’ label had not been updated for many years and it was time for the labels to reflect the current science and nutrient needs of Americans. With the support of its members and a creative outside agency, CRN has developed a campaign that accomplishes many goals, in particular, communicating that Supplement Facts labels are changing to better provide consumers the information they need to make informed choices about their health (2).
Wommack also addresses the need to ease consumers’ minds about the label changes. CRN wants consumers to value the helpfulness of updated labels. As a result, CRN launched this educational campaign. Moreover, the organization hopes industry leaders and key stakeholders will share and spread the Be Label Wise campaign.
Also, even though anyone can use the educational content in stakeholder toolkit, the tools can also be customized and branded. CRN also has social media tools with graphics with text to help the campaign reach even more people.
Additionally, Wommack states that 75% of Americans take dietary supplements. He then sees these upcoming changes as a great opportunity for the nutritional supplement industry to educate consumers.

Specific FDA Supplement Facts Label Changes
According to the new FDA’s new labeling guidelines, below are specific changes consumers will see on the new labels (3):
- New Daily Values (DVs) — will be based on changes in the American diet and updated nutrition science
- Vitamin A, D, and E amounts — will change from International Units (IU) to milligrams (mg) and micrograms (mcg)
- Folic acid — will now be listed as folate and measured in mcg of dietary folate equivalents or DFEs
- Added sugar — Updated labels will contain amount and % DV of added sugar
The FDA’s changes will go into effect in 2020 and 2021. Manufacturers who have over $10 million in annual sales must comply by January 1, 2020. For manufacturers with less than $10 million in annual sales, they have until by January 1, 2021, to comply with the new guidelines (4).
At the same time, the Be Label Wise campaign points out that the compliance date is based on when the product is actually labeled, not according to shipping or shelving dates. Furthermore, some companies may even change their labels well in advance of the deadlines.

FDA Supplement Facts Labels History and the Need for Changes
After passing the Dietary Supplement Health and Education Act of 1994 (DSHEA), the FDA’s Supplement Facts became a part of federal regulations in 1997. The FDA amended these guidelines the following year and once again in 2003.
Located on dietary supplements, the FDA created the Supplement Facts label to inform consumers about the nutrient make-up of nutritional products. According to FDA regulations, each label must list the ingredients, serving size, % Daily Value (DV), the product’s suggested use, and other important information that consumers should know.
Since the previous updates happened over a decade and a half ago, the FDA decided to make changes. Two major factors led to these changes to Supplement Facts labels. Firstly, the average American diet has changed in the past few decades. Secondly, there have been many advances in nutrition science. Facing these two things, the FDA now asks supplement companies to update their labels based on the new guidelines.
About CRN
Since 1973, this Washington, D.C.-based trade association has been the leading trade association for the dietary supplement industry. CRN represents over 150 companies in the industry, from suppliers to manufacturers. CRN expects all members to follow all relevant federal and state regulations regarding quality control, manufacturing, marketing, and safety. Furthermore, CRN members must also adhere to voluntary guidelines and CRN’s Code of Ethics.
References

